U.S. judge Sidney Stein today granted a request from Sanofi-Aventis and Bristol-Myers Squibb Co. to block the sale of a generic form of their top-selling Plavix blood thinner.
However, the federal judge denied a motion seeking a recall of the generic product that has already been distributed by Canadian drugmaker Apotex.
BMS shares rose nearly 7 percent in extended trade.
Fox
Plavix, the No. 2-selling prescription medicine in the world after the cholesterol drug Lipitor, has worldwide sales of more than $6 billion.
Brand-name sales in the United States plummeted last week to $14 million -- down from $62.7 million a week before Apotex's launch -- as generic Plavix seized 75 percent of new prescriptions, according to Verispan, a consumer market-research company.
Stein's order remains in effect until a patent-infringement suit by Bristol-Myers and Sanofi is heard or Apotex is successful in having the injunction overturned by a federal appeals court.
"It's stunning," Barry Sherman, Apotex's founder and chief executive, said in a telephone interview from the company's headquarters in suburban Toronto. "We feel there is strong grounds for appeal and will appeal immediately."
Those of you who want to see Judge Stein's entire ruling can go to this link:
http://www.nysd.uscourts.gov/courtweb/public.htm
Looking beyond the spin of Big Pharma PR. But encouraging gossip. Come in and confide, you know you want to! “I’ll publish right or wrong. Fools are my theme, let satire be my song.” Email: jackfriday2011(at)hotmail.co.uk
Thursday, August 31, 2006
One Air Pfizer - the only way to fly
Welcome to Air Pfizer
Buckle up and enjoy!
Your pilot: Dr Peter Rost
First Officer: Cap'n John Mack
Beef or Chicken?
Buckle up and enjoy!
Your pilot: Dr Peter Rost
First Officer: Cap'n John Mack
Beef or Chicken?
Pfizer vs Ranbaxy - Lipitor: Goliath vs David
Patent protection of the world's biggest selling drug, Pfizer's $12-billion a year cholesterol-buster Lipitor, has taken a hit in Norway, after a court ruled in favour of challenger Ranbaxy Laboratories in its fight to sell a copycat version.
The Oslo City Court decided that Ranbaxy's generic version of Lipitor (atorvastatin) does not infringe on two of the branded drug's patents, which follows a similar ruling on a third patent earlier this year.But towards the end of 2005, the court ruled that a fourth patent was being infringed upon, thereby preventing the Indian drugmaker from launching its product until this expires in February 2009.
Therefore, this latest ruling has "no practical effect" on Lipitor's current protection in Norway, Pfizer said in a statement, adding that in other European markets, the drug is protected until 2011.
Ranbaxy is fighting hard to sell its generic version of Lipitor around the globe. Earlier this month, a US court backed the validity of the main patent protecting the drug, although it ruled a second patent invalid on technical grounds.
This basically means that Ranbaxy can launch a copycat in the US in 2010 rather than 2011.
Ranbaxy is among those generic companies that adopt a high-risk, high-reward strategy in the sector, spending on litigation in the hope of winning first-to-market advantage for its generic products.
No wonder Pfizer's new CEO is a lawyer!
Source: PharmaTimes
The Oslo City Court decided that Ranbaxy's generic version of Lipitor (atorvastatin) does not infringe on two of the branded drug's patents, which follows a similar ruling on a third patent earlier this year.But towards the end of 2005, the court ruled that a fourth patent was being infringed upon, thereby preventing the Indian drugmaker from launching its product until this expires in February 2009.
Therefore, this latest ruling has "no practical effect" on Lipitor's current protection in Norway, Pfizer said in a statement, adding that in other European markets, the drug is protected until 2011.
Ranbaxy is fighting hard to sell its generic version of Lipitor around the globe. Earlier this month, a US court backed the validity of the main patent protecting the drug, although it ruled a second patent invalid on technical grounds.
This basically means that Ranbaxy can launch a copycat in the US in 2010 rather than 2011.
Ranbaxy is among those generic companies that adopt a high-risk, high-reward strategy in the sector, spending on litigation in the hope of winning first-to-market advantage for its generic products.
No wonder Pfizer's new CEO is a lawyer!
Source: PharmaTimes
Pfizer - Jeff Kindler is Homer Simpson in "Catch me if you can!"
Pfizer - Jeff Kindler is Homer Simpson in "Catch me if you can!"
Dr. Peter Rost's excellent blog is on a roll!
He is chasing down the facts on the Pfizer company jets and their use by the new CEO Jeff Kindler and his family.
LOL!
Dr. Peter Rost's excellent blog is on a roll!
He is chasing down the facts on the Pfizer company jets and their use by the new CEO Jeff Kindler and his family.
LOL!
MDs and Big Pharma - time for a D.I.V.O.R.C.E
Pennie Marchetti, MD says Divorce Ourselves From Drug Companies
Keeping up with the latest innovations in drug therapy can certainly be a challenge. Many times, patients hear about new developments from the media before we lowly physicians learn of them. It's not uncommon for my patients to ask for a drug that's just been approved by the FDA the day before — long before it makes it to the marketplace, let alone my own knowledge base.
So, what's a busy physician to do?
First of all, let's start with what not to do.
Do not rely entirely on pharmaceutical sales representatives, any more than you would rely on a car salesman for information about a car. Pharmaceutical sales reps, and the companies who hire them, have done a good job of convincing themselves (as well as the public and some doctors) that one of their key missions is to educate physicians.
Don't believe them.
Their key mission is to close a sale.
As the Harvard Business Review reports, the new CEO of Schering-Plough credits his company's turnaround to "motivating and organizing salespeople to create trusting relationships with doctors."[1] The information they provide is often inaccurate,[2,3] and our ability as a group to sort the misleading information from the valid is abysmally poor.[2] It should be no surprise, then, that the more pharmaceutical reps a physician sees, the poorer his prescribing habits will be.[4]
That leaves us with the medical experts — those doctors who are the leaders in their fields, who give lectures at conferences, who publish papers in respected journals, and who write guidelines. But not all experts are created equal. Too many of them have financial ties to pharmaceutical companies that rival those of the sales reps. Recent examples include the influence of drug company money on defining hypertension[5] and on classifying psychiatric illnesses.[6]
Even patient advocacy groups are financially intertwined with the pharmaceutical industry, and not just in the form of grants or donations. Executives from Eli Lilly, for example, recently gave the American Diabetes Association a helping hand with its business strategy.[7]
Such coziness certainly gives one pause when mulling over new treatment guidelines.
What about journals? The New England Journal of Medicine, JAMA, and other professional society journals can be important sources of original studies about drugs. In fact, they are the only source we have to fact-check the experts and the drug reps. However, these too must be approached with a healthy skepticism and an eye for industry bias, not only from authors of drug industry-funded studies but from the editors, too.
Consider the case of Redux, the antiobesity drug that benefited from a very favorable editorial in The New England Journal of Medicine. There were reports in 1996 that Europeans who used the drug were suffering from higher than usual rates of pulmonary hypertension,[8] but the editorial argued that the weight loss facilitated by the drug outweighed its associated pulmonary risks.[9]
With such a ringing endorsement from such a respected source, the drug's sales skyrocketed.
Just a year later, however, there were more reports of fatal pulmonary hypertension[10] and of heart disease[11] associated with the drug.
It was removed from the market.
And The New England Journal of Medicine's respected editorialists?
As it turns out, they were in the employ of the diet pill industry.[12] To the journal's credit, it did publish all of the original articles documenting the drug's dangers, but lax editorial standards contributed to the widespread adoption of a drug of very little benefit at substantial risk.
That doesn't leave us with much in the way of reliable sources, does it? Who has the time to look up every study on newly introduced drugs, let alone analyze them carefully? Luckily, there are some reliable sources remaining, sources that do the analyzing for us. One of those is The Medical Letter, which reviews the claims of new drugs and the research behind those claims (published and unpublished) in an unbiased fashion. Not coincidentally, it doesn't accept advertising. Another good source is the Cochrane Reviews, a database of evidence-based assessments of various interventions, including drugs. Both require a subscription, but the American Academy of Family Physicians runs a feature in its monthly journal summarizing various Cochrane Reviews,[13] available online for free, even to nonmembers.
It's rather sad that there's more wrong than right with the current state of physician pharmacologic education. Obviously, as a profession, we could do better. And the place to start is by divorcing ourselves from drug company money.
Source: MedScape Today
Keeping up with the latest innovations in drug therapy can certainly be a challenge. Many times, patients hear about new developments from the media before we lowly physicians learn of them. It's not uncommon for my patients to ask for a drug that's just been approved by the FDA the day before — long before it makes it to the marketplace, let alone my own knowledge base.
So, what's a busy physician to do?
First of all, let's start with what not to do.
Do not rely entirely on pharmaceutical sales representatives, any more than you would rely on a car salesman for information about a car. Pharmaceutical sales reps, and the companies who hire them, have done a good job of convincing themselves (as well as the public and some doctors) that one of their key missions is to educate physicians.
Don't believe them.
Their key mission is to close a sale.
As the Harvard Business Review reports, the new CEO of Schering-Plough credits his company's turnaround to "motivating and organizing salespeople to create trusting relationships with doctors."[1] The information they provide is often inaccurate,[2,3] and our ability as a group to sort the misleading information from the valid is abysmally poor.[2] It should be no surprise, then, that the more pharmaceutical reps a physician sees, the poorer his prescribing habits will be.[4]
That leaves us with the medical experts — those doctors who are the leaders in their fields, who give lectures at conferences, who publish papers in respected journals, and who write guidelines. But not all experts are created equal. Too many of them have financial ties to pharmaceutical companies that rival those of the sales reps. Recent examples include the influence of drug company money on defining hypertension[5] and on classifying psychiatric illnesses.[6]
Even patient advocacy groups are financially intertwined with the pharmaceutical industry, and not just in the form of grants or donations. Executives from Eli Lilly, for example, recently gave the American Diabetes Association a helping hand with its business strategy.[7]
Such coziness certainly gives one pause when mulling over new treatment guidelines.
What about journals? The New England Journal of Medicine, JAMA, and other professional society journals can be important sources of original studies about drugs. In fact, they are the only source we have to fact-check the experts and the drug reps. However, these too must be approached with a healthy skepticism and an eye for industry bias, not only from authors of drug industry-funded studies but from the editors, too.
Consider the case of Redux, the antiobesity drug that benefited from a very favorable editorial in The New England Journal of Medicine. There were reports in 1996 that Europeans who used the drug were suffering from higher than usual rates of pulmonary hypertension,[8] but the editorial argued that the weight loss facilitated by the drug outweighed its associated pulmonary risks.[9]
With such a ringing endorsement from such a respected source, the drug's sales skyrocketed.
Just a year later, however, there were more reports of fatal pulmonary hypertension[10] and of heart disease[11] associated with the drug.
It was removed from the market.
And The New England Journal of Medicine's respected editorialists?
As it turns out, they were in the employ of the diet pill industry.[12] To the journal's credit, it did publish all of the original articles documenting the drug's dangers, but lax editorial standards contributed to the widespread adoption of a drug of very little benefit at substantial risk.
That doesn't leave us with much in the way of reliable sources, does it? Who has the time to look up every study on newly introduced drugs, let alone analyze them carefully? Luckily, there are some reliable sources remaining, sources that do the analyzing for us. One of those is The Medical Letter, which reviews the claims of new drugs and the research behind those claims (published and unpublished) in an unbiased fashion. Not coincidentally, it doesn't accept advertising. Another good source is the Cochrane Reviews, a database of evidence-based assessments of various interventions, including drugs. Both require a subscription, but the American Academy of Family Physicians runs a feature in its monthly journal summarizing various Cochrane Reviews,[13] available online for free, even to nonmembers.
It's rather sad that there's more wrong than right with the current state of physician pharmacologic education. Obviously, as a profession, we could do better. And the place to start is by divorcing ourselves from drug company money.
Source: MedScape Today
Pfizer - Celebrex: making a silk purse out of a sows' ear

Poor Pfizer.
Despite Vioxx, they have not given up on their coxib painkiller Celebrex.
They must be desperate for products to promote!
Annual sales are expected to top $2 billion this year after falling to $1.7 billion last year. Sales were at $3.3 billion in 2004.
Yet, the likelihood that Celebrex will again be a $2 billion-a-year drug surprises Sharon Levine, a physician and associate executive director of the 6,400-member doctor group that cares for Kaiser Permanente customers in Northern California.
"I'm stunned," she says. "Given its modest benefit as a pain reliever and its modest theoretical benefit for gastrointestinal protection ... it's an expensive choice in a category where there are many others."
Not many Kaiser patients were on COX-2s before the Vioxx withdrawal, she says. Now, Kaiser doctors put even fewer patients, less than 1%, on Celebrex, she says.
Nationwide, Celebrex captured 9.3% of new prescriptions among arthritis drug users in July, says market researcher Verispan.
More at USA Today
Sanofi Aventis - Brooksby "bigs up" Acomplia in the UK

Sanofi-Aventis' UK MD says they have seen "strong demand" for new obesity pill Acomplia since its launch in Britain in late June.
Nigel Brooksby said that although NICE, which assesses the cost effectiveness of new drugs, was unlikely to give a verdict on Acomplia before the first half of 2008, many doctors were already eager to prescribe it.
"We're very pleased. The UK launch has certainly met our expectations," he said in a telephone interview. "The qualitative information we are receiving is that it is getting a very good reception."
Independent prescription data measuring the drug's uptake are not yet available.
More from Ben Hirschler at Reuters
Insider's view: Let's wait and see the "figures"!
Remember some (ex) Big Pharma CEO's think the UK market is a "dog"!
Wednesday, August 30, 2006
Bonnie Raitt - Angel from Montgomery
Bonnie Raitt - Angel from Montgomery
http://www.youtube.com/watch?v=Ihpi8yBwLe8
A beautiful John Prine song.
Strong good memories. For Petite le Hat and friends on that little mound of rock.
http://www.youtube.com/watch?v=Ihpi8yBwLe8
A beautiful John Prine song.
Strong good memories. For Petite le Hat and friends on that little mound of rock.
Merck - Vioxx: yet another retrial!
A federal judge has overturned a $50 million jury award against Merck in a lawsuit filed by a Vioxx user.
Judge Eldon Fallon ruled that the damage award in the federal Vioxx case this month was excessive, and that a new trial must be held on the damages for a retired FBI agent who suffered a heart attack after taking the painkiller, the Associated Press reported.
Nice (and more) work for the lawyers. Kerching!
Judge Eldon Fallon ruled that the damage award in the federal Vioxx case this month was excessive, and that a new trial must be held on the damages for a retired FBI agent who suffered a heart attack after taking the painkiller, the Associated Press reported.
Nice (and more) work for the lawyers. Kerching!
Public Citizen are hopping mad over tendon ruptures with quinolones

US consumer group, Public Citizen, is on the warpath again and in its line of sight this time are the fluoroquinolone antibiotics Levaquin and Cipro, which it says have been linked to ruptured tendons.
Public Citizen has petitioned the FDA along with the attorney general for the State of Illinois, Lisa Madigan, saying there were around 260 cases each of tendon ruptures, tendonitis and other tendon disorders in an eight-year period to the end of 2005.
The large majority, 61%, were linked to the use of Johnson & Johnson's Levaquin (levafloxacin) while a large proportion of the remainder - 23% - were associated with Bayer's Cipro (ciprofloxacin)'s use (although the latter is also available generically).
"The numbers are startling. Tendon ruptures associated with these drugs continue to occur at a disturbing rate but could be prevented if doctors and patients were more aware of early warning signals, such as the onset of tendon pain, and switched to other antibiotics," argued Sidney Wolfe, director of Public Citizen's Health Research Group.
It believes that the fluoroquinolone group of drugs are toxic to tendon fibres and may cut the supply of blood causing the tendon to rupture. Most commonly, the Achilles' tendon has ruptured in patients taking the antibiotics, leading to severe pain and difficulty walking, but other sites reported include the biceps, the hand and the thumb. Although the labelling already does warn of tendon ruptures following a 1996 Public Citizen petition, it is "buried in the list of possible adverse reactions," said the consumer group, "and so has been inadequate."
The Illinois attorney general followed this up in 2005 with a call for a black box warning - the strongest available - but said the FDA "has never responded substantively." If more doctors and patients were aware of the early warning signals - such as the onset of tendon pain - they could be switched to other antibiotics, the group says.
"The numbers are startling. Tendon ruptures associated with these drugs continue to occur at a disturbing rate, but could be prevented. The FDA must act and require black box warnings and patient information guides," Public Citizen finishes. Some 45% of all prescriptions for fluoroquinolone antibiotics over the last four years were written for Levaquin.
Source: PharmaTimes
Wyeth - Effexor XR: is depression looming?
Poor Wyeth. Just when they thought things were going swimmingly for their follow up "evergreener" to Effexor XR (venlafaxine).
The follow up drug, desvenlafaxine (an isomer of the parent, a la Nexium vs Prilosec for AstraZeneca's PPIs) has been delayed by three months by the FDA.
The FDA had been expected to decide on the new antidepressant by October. But Wyeth said the agency has delayed the deadline until January 22, 2007, in order to study recently submitted pre-clinical data on whether the drug increases the risk of cancer.
Reuters.
Insider's view: could be somthing, could be nothing.
Given that half of Effexor XR is desvenlafaxine (one of two isomers of venlafaxine), this cancer scare must be worrying for the Effexor XR Brand Manager!
The follow up drug, desvenlafaxine (an isomer of the parent, a la Nexium vs Prilosec for AstraZeneca's PPIs) has been delayed by three months by the FDA.
The FDA had been expected to decide on the new antidepressant by October. But Wyeth said the agency has delayed the deadline until January 22, 2007, in order to study recently submitted pre-clinical data on whether the drug increases the risk of cancer.
Reuters.
Insider's view: could be somthing, could be nothing.
Given that half of Effexor XR is desvenlafaxine (one of two isomers of venlafaxine), this cancer scare must be worrying for the Effexor XR Brand Manager!
Sanofi Aventis - Plavix: Germans say "nein"!
German health insurers, under pressure to cut costs amid reforms, are considering whether to restrict prescription guidelines for Sanofi-Aventis's blood thinner Plavix in a move that could harm the drug's sales.
The German Institute for Quality and Efficiency in Healthcare (IQWiG) said clopidogrel, marketed by Sanofi-Aventis and Bristol-Myers Squibb as Plavix or Iscover, offered no benefits over aspirin when used alone as a preventative treatment for conditions resulting from arterial diseases.
Source
The German Institute for Quality and Efficiency in Healthcare (IQWiG) said clopidogrel, marketed by Sanofi-Aventis and Bristol-Myers Squibb as Plavix or Iscover, offered no benefits over aspirin when used alone as a preventative treatment for conditions resulting from arterial diseases.
Source
Schering Plough - conspiracy: guilty as charged!

"With this agreement, we are putting issues from the past behind us," said Brent Saunders, senior vice president for compliance and business practices.
What issues might those be, Brent?
Schering-Plough has agreed to pay $435 million and plead guilty to conspiracy to settle a federal investigation into marketing of its drugs for unapproved uses and overcharging Medicaid for certain drugs.
They will pay $255 million to resolve civil aspects of the previously disclosed investigation and will also pay a criminal fine of $180 million and plead guilty to one count of conspiracy to make false statements to the government.
The agreement is subject to court approval.
The Department of Justice said that the inquiry covered illegal sales and marketing practices for the brain cancer drug Temodar and the bladder cancer and hepatitis treatment Intron A. It also addressed allegations of fraud involving Medicaid, the healthcare scheme, over pricing for the company’s Claritin RediTabs allergy medicine and K-Dur for stomach conditions.
U.S. Attorney Michael J. Sullivan, who announced the settlement, said health care corruption "erodes public confidence, compromises the patient-physician relationship and adds costs to important government programs."
"The American people, as both taxpayers and consumers, expect our health care system to be free from fraud and corruption," Sullivan said.
As part of the settlement, Schering Plough has said it would add a section to an existing corporate integrity agreement it has with the Department of Health and Human Services. The agreement requires the company to monitor its sales, marketing and drug pricing, and to correct past abuses.
More at the WaPo.
Insider's view: it's a start. The stables must be cleaned. Grab a broom Brent!
Tuesday, August 29, 2006
Casting call - MD wanted for The Discovery Channel
They're looking for a youthful and dynamic physician to be part of a team of hosts for a new Discovery Channel popular science television series - think "Mythbusters" meets the human body.
Our ideal candidate for the position:
A Doctor with knowledge of the latest medical research who is comfortable speaking about a range of disciplines.
Able to communicate complicated human biological and medical concepts to an adult audience with a general science education - a teaching background is a plus.
Capable of devising unique and entertaining physical demonstrations that help illustrate the inner workings of the human body.
Has a sense of humor, is a team player, and is comfortable in front of a video camera.
Available to travel for filming a few days a month for the next six months starting this Fall.
If you are interested in being considered, please email the following information to: CYoung@granadausa.com:
Your name and contact info (email, phone #, and website, if any)
A recent photo
Your resume
A brief paragraph stating your interest and qualifications for the hosting position. Please summarize your background and detail any additional relevant information not reflected in your resume.
Our ideal candidate for the position:
A Doctor with knowledge of the latest medical research who is comfortable speaking about a range of disciplines.
Able to communicate complicated human biological and medical concepts to an adult audience with a general science education - a teaching background is a plus.
Capable of devising unique and entertaining physical demonstrations that help illustrate the inner workings of the human body.
Has a sense of humor, is a team player, and is comfortable in front of a video camera.
Available to travel for filming a few days a month for the next six months starting this Fall.
If you are interested in being considered, please email the following information to: CYoung@granadausa.com:
Your name and contact info (email, phone #, and website, if any)
A recent photo
Your resume
A brief paragraph stating your interest and qualifications for the hosting position. Please summarize your background and detail any additional relevant information not reflected in your resume.
FDA - Expert panels are "rubber stamps"
The U.S. Food and Drug Administration's outside advisory panels typically serve as "rubber stamps" for companies seeking approval of drugs and medical devices.
Eleven randomly selected FDA advisory committees recommended approval in 79 percent of their votes on applications between 1998 and 2005, according to the study released Monday by the Washington-based National Research Center for Women & Families. The committees considered 89 products during that period.
"Some of the committees have never met a product they don't like," said Diana Zuckerman, president of the group, said in a telephone interview.
The FDA said in July that it plans to write new guidelines specifying when scientists and doctors serving on the panels should be disqualified because of conflicts of interest. Under FDA rules, the agency can grant waivers for those with conflicts, allowing them to serve on panels.
More.
Insider's view: hear hear! Expert panels are rubber stamp committees who can share the blame if anything goes wrong.
Could it possibly have somthing to do with the financial links so many of these "experts" have with Big Pharma?
And are these "experts" upto the job anyway?
Take Pargluva, for example, didn't the expert panel approve that!?
Eleven randomly selected FDA advisory committees recommended approval in 79 percent of their votes on applications between 1998 and 2005, according to the study released Monday by the Washington-based National Research Center for Women & Families. The committees considered 89 products during that period.
"Some of the committees have never met a product they don't like," said Diana Zuckerman, president of the group, said in a telephone interview.
The FDA said in July that it plans to write new guidelines specifying when scientists and doctors serving on the panels should be disqualified because of conflicts of interest. Under FDA rules, the agency can grant waivers for those with conflicts, allowing them to serve on panels.
More.
Insider's view: hear hear! Expert panels are rubber stamp committees who can share the blame if anything goes wrong.
Could it possibly have somthing to do with the financial links so many of these "experts" have with Big Pharma?
And are these "experts" upto the job anyway?
Take Pargluva, for example, didn't the expert panel approve that!?
Monday, August 28, 2006
How Wyeth beat Astra in their own back yard - by Dr Peter Rost
The good doctor is telling his own work/life story on his blog at the moment.
Well worth a read.
Here's a great piece about how he (when he was MD of the Nordic Region) and his team whooped Nexium (esomeprazole) and AstraZeneca's ass with Prevacid (lansoprazole).
"So I figured if the carrot doesn’t work, maybe a machine gun will, like forcing them (Swedish doctors) to use the new drug through low prices and mandatory formularies. That worked. The Swedish doctors were forced to change their wasteful ways.
The rest is pharmaceutical history in Europe. (And today they have generic substitution, further putting pressure on drug costs). Sales took off like crazy. Within a year we had about 30% market share in value and close to 50% in volume.
Astra was being killed in their own home market. And the press was helping us. Because this is when I learned to work with the press, not against it, the way most pharma companies in reality do. (Some virtually refused to respond to press inquiries ten years ago.) And the press was having a field day, because here was this tiny American company beating the sh-t out of one of Sweden’s crown jewels and the Swedish business establishment.
Boy, were “they” pissed. I even got called in to private meetings where "they" tried to make me stop. I told them my share holders were happy and asked if they had a problem with their own share holders. They didn’t think that was funny.
However, Insider thinks it's hilarious!
Well worth a read.
Here's a great piece about how he (when he was MD of the Nordic Region) and his team whooped Nexium (esomeprazole) and AstraZeneca's ass with Prevacid (lansoprazole).
"So I figured if the carrot doesn’t work, maybe a machine gun will, like forcing them (Swedish doctors) to use the new drug through low prices and mandatory formularies. That worked. The Swedish doctors were forced to change their wasteful ways.
The rest is pharmaceutical history in Europe. (And today they have generic substitution, further putting pressure on drug costs). Sales took off like crazy. Within a year we had about 30% market share in value and close to 50% in volume.
Astra was being killed in their own home market. And the press was helping us. Because this is when I learned to work with the press, not against it, the way most pharma companies in reality do. (Some virtually refused to respond to press inquiries ten years ago.) And the press was having a field day, because here was this tiny American company beating the sh-t out of one of Sweden’s crown jewels and the Swedish business establishment.
Boy, were “they” pissed. I even got called in to private meetings where "they" tried to make me stop. I told them my share holders were happy and asked if they had a problem with their own share holders. They didn’t think that was funny.
However, Insider thinks it's hilarious!
Wyeth - Prempro trial: red flags, I see no red flags
 From the first Wyeth Prempro HRT trial in Little Rock, Arkansas:
From the first Wyeth Prempro HRT trial in Little Rock, Arkansas:A 20-year veteran of the U. S. Food and Drug Administration testified Saturday about more than a dozen “red flags” that popped up in the last 30 years alerting Wyeth Pharmaceuticals to potential links between its hormone drugs and breast cancer.
Dr. John Gueriguian, an endocrinologist who was a medical director at the FDA from 1978 through 1998, testified Saturday that the endometrial cancer study “absolutely” was “a signal that the company should study what its drug is doing to the rest of the body.”
“It would make sense to begin investigating the next organ that responds to hormones,” he said.
Gueriguian, who approved and monitored new hormone drugs for the FDA, testified that signals indicating potential problems with new drugs often arise slowly.
“If all the blips are pointing in the same direction, if they add up, a drug company should say, ‘We have something that can’t be ignored, ’” he said.
Indeed, an internal Wyeth memo dated March 12, 1980, showed that an outside expert told Wyeth officials that “a well-founded, long-range and controlled epidemiological investigation” must be done to ascertain the effect of Premarin (Prempro minus the progestin) on breast cancer.
It wasn’t until a NIH funded study on Prempro’s potential cardiovascular benefits revealed a 24 percent increase in breast cancer in the women who took it, as opposed to those who took a placebo, that prescriptions for the drug dropped dramatically.
Wyeth are facing around 5,000 such lawsuits.
Source.
Top ten reasons why there are more women in PR
With apologies to Strumpette:
#10... Because in PR the ass you have to kiss is (usually) clean.
#9… Cause they dream of meeting Oprah or Uma or Tom or Brad. Shame they never will.
#8... Cause Mr. Weatherwax seems to like my bubblegum-pink plunging tanktop.
#7... Cause the boys don’t want to take the time to learn the cheers.
#6… Cause you can get a decent amount of work out of them for 3 out of every 4 weeks. More than guys.
#5... "They promised to let me clean the executive washroom."
#4... Cause, generally speaking, women make better slaves.
#3… Higher chance of becoming a CEO's wife/mistress/girlfriend (considered career progression) than most jobs.
#2... Heart-wrenching display of brown-nosing offers unique insight into pathos of the female human condition.
And the #1 Reason there’s so many women in PR… ‘cause GIRLS LIE!
#10... Because in PR the ass you have to kiss is (usually) clean.
#9… Cause they dream of meeting Oprah or Uma or Tom or Brad. Shame they never will.
#8... Cause Mr. Weatherwax seems to like my bubblegum-pink plunging tanktop.
#7... Cause the boys don’t want to take the time to learn the cheers.
#6… Cause you can get a decent amount of work out of them for 3 out of every 4 weeks. More than guys.
#5... "They promised to let me clean the executive washroom."
#4... Cause, generally speaking, women make better slaves.
#3… Higher chance of becoming a CEO's wife/mistress/girlfriend (considered career progression) than most jobs.
#2... Heart-wrenching display of brown-nosing offers unique insight into pathos of the female human condition.
And the #1 Reason there’s so many women in PR… ‘cause GIRLS LIE!
Neuropsychopharmacology Journal - the ties that bind
The editor of the journal Neuropsychopharmacology is stepping down following a flap over the medical journal's failure to disclose that the authors of a paper reviewing a new treatment for depression had financial ties to the treatment's developer.
One of the authors of the article was the editor himself, Charles B.Nemeroff, who is the chairman of the Department of Psychiatry and Behavioral Sciences at Emory University in Atlanta.
In an email Friday, the owner of the medical journal said Dr. Nemeroff had decided to step down as editor. They said his decision was "in part, based on the recent adverse publicity to the journal."
More
One of the authors of the article was the editor himself, Charles B.Nemeroff, who is the chairman of the Department of Psychiatry and Behavioral Sciences at Emory University in Atlanta.
In an email Friday, the owner of the medical journal said Dr. Nemeroff had decided to step down as editor. They said his decision was "in part, based on the recent adverse publicity to the journal."
More
Sunday, August 27, 2006
"One Pfizer" - Kindler's halo effect

“There is definitely a halo effect (on Kindler),” said David Moskowitz, an analyst at Friedman, Billings, Ramsey. “But investors are impatient. The honeymoon will be short.”
More of Kindler's PR "charm offensive" here.
He will have to get a move on sorting out his "to do" list.
The US Exubera launch must be weighing heavily on his mind.
This product is not a guaranteed success by any means!
Saturday, August 26, 2006
GOP - Bought and paid for by Big Pharma
U.S. Rep. Thelma Drake was grateful for the TV campaign ads praising her support for the new and much-criticized federal Medicare Part D prescription drug program for seniors.
You know, the program that forbids Medicare from negotiating price discounts from Big Pharma.
Her campaign manager thanked the national chamber of commerce for airing them on her behalf over the last few weeks.
It turns out the pharmaceutical industry actually paid to air the ads as part of a nationwide $10 million covert campaign to help some GOP incumbents, according to The Associated Press, citing unnamed political officials.
Democrats are howling in outrage at the revelation.
"This Republican Congress looks out for the big drug companies, and the big drug companies return the favor by pouring special interest money into local campaigns," said Kate Bedingfield, spokeswoman for the Democratic Congressional Campaign Committee.
"Virginia seniors are tired of taking a back seat to corporate profits and they want a representative who will fight for their bottom line for a change."
So, Big Pharma quietly footed the bill for at least part of a recent multimillion-dollar ad campaign praising lawmakers who support the new Medicare program.
The U.S. Chamber of Commerce claims credit for the ads, although a spokesman refused repeatedly to say whether it had received any funds from the Pharmaceutical Research and Manufacturers of America.
Drake's opponent, Democrat Phil Kellam, on Friday blasted Drake and the ad campaign that benefited her re-election effort.
"This is a desperate attempt to protect a crony," he said. "They're investing in Thelma Drake. Just as she's grafted to George Bush, she's grafted to the drug industry. This is a convoluted, clandestine attempt to mislead the voters and people in general."
More here and here.
You know, the program that forbids Medicare from negotiating price discounts from Big Pharma.
Her campaign manager thanked the national chamber of commerce for airing them on her behalf over the last few weeks.
It turns out the pharmaceutical industry actually paid to air the ads as part of a nationwide $10 million covert campaign to help some GOP incumbents, according to The Associated Press, citing unnamed political officials.
Democrats are howling in outrage at the revelation.
"This Republican Congress looks out for the big drug companies, and the big drug companies return the favor by pouring special interest money into local campaigns," said Kate Bedingfield, spokeswoman for the Democratic Congressional Campaign Committee.
"Virginia seniors are tired of taking a back seat to corporate profits and they want a representative who will fight for their bottom line for a change."
So, Big Pharma quietly footed the bill for at least part of a recent multimillion-dollar ad campaign praising lawmakers who support the new Medicare program.
The U.S. Chamber of Commerce claims credit for the ads, although a spokesman refused repeatedly to say whether it had received any funds from the Pharmaceutical Research and Manufacturers of America.
Drake's opponent, Democrat Phil Kellam, on Friday blasted Drake and the ad campaign that benefited her re-election effort.
"This is a desperate attempt to protect a crony," he said. "They're investing in Thelma Drake. Just as she's grafted to George Bush, she's grafted to the drug industry. This is a convoluted, clandestine attempt to mislead the voters and people in general."
More here and here.
Friday, August 25, 2006
Barr - Plan B: the creation of a "behind the counter" category
Emergency hormonal contraception has been sold by UK pharmacists for some time now.
The system works well. It is a "P" (for Pharmacy) category medicine. The pharmacist "must be able to intervine" in the sale of a P medicine.
Now the US, as a side effect of Plan B's approval look like they are creating the same kind of thing.
Under the FDA’s approval of the drug, Plan B will be kept behind the counter at pharmacies; customers will have to show proof of age in order to purchase it. In addition the drug will only be supplied by wholesalers to outlets that have a pharmacist available for consultation.
Sounds sensible.
Now what other prescription medicines could be sold in a similar manner?
The system works well. It is a "P" (for Pharmacy) category medicine. The pharmacist "must be able to intervine" in the sale of a P medicine.
Now the US, as a side effect of Plan B's approval look like they are creating the same kind of thing.
Under the FDA’s approval of the drug, Plan B will be kept behind the counter at pharmacies; customers will have to show proof of age in order to purchase it. In addition the drug will only be supplied by wholesalers to outlets that have a pharmacist available for consultation.
Sounds sensible.
Now what other prescription medicines could be sold in a similar manner?
Roche - Herceptin: NICE OK's use in early breast cancer
Proof that they can and do say "yes" sometimes.
The National Institute of Health and Clinical Excellence (NICE) has published a technology appraisal on the use of Herceptin in early breast cancer.
NICE recommend that it is given to women with early stage HER2-positive breast cancer after they have had surgery and chemotherapy (and radiotherapy if appropriate). It should be given every three weeks for a period of twelve months or until the breast cancer returns, which ever is sooner, provided that the patient has good cardiac function.
Before treatment is started cardiac function should be assessed. Treatment should not be offered if the patient has:
a left ventricular ejection fraction (LVEF) of 55% or less
a history of documented congestive heart failure
high-risk uncontrolled arrhythmias
angina pectoris requiring medication
clinically significant valvular disease
evidence of transmural infarction on electrocardiograph (ECG)
poorly controlled hypertension
NICE also recommend that cardiac assessments are carried out every 3 months and treatment suspended if LVEF drops below 50% or by 10% from the baseline.
Source: Prescribing Advice for GPs.
Insider's view: NICE have made some difficult decisions recently, both positive and negative. IMHO they have been the correct ones.
The National Institute of Health and Clinical Excellence (NICE) has published a technology appraisal on the use of Herceptin in early breast cancer.
NICE recommend that it is given to women with early stage HER2-positive breast cancer after they have had surgery and chemotherapy (and radiotherapy if appropriate). It should be given every three weeks for a period of twelve months or until the breast cancer returns, which ever is sooner, provided that the patient has good cardiac function.
Before treatment is started cardiac function should be assessed. Treatment should not be offered if the patient has:
a left ventricular ejection fraction (LVEF) of 55% or less
a history of documented congestive heart failure
high-risk uncontrolled arrhythmias
angina pectoris requiring medication
clinically significant valvular disease
evidence of transmural infarction on electrocardiograph (ECG)
poorly controlled hypertension
NICE also recommend that cardiac assessments are carried out every 3 months and treatment suspended if LVEF drops below 50% or by 10% from the baseline.
Source: Prescribing Advice for GPs.
Insider's view: NICE have made some difficult decisions recently, both positive and negative. IMHO they have been the correct ones.
Thursday, August 24, 2006
JAMA - DeAngelis tries to "clean house"
JAMA's editor Catherine DeAngelis writes an editorial " The Influence of Money on Medical Science":
Now comes the potential problem. In some instances, the marketing goal of a company dominates the scientific aspect of the company-funded research. There have been a number of high-profile examples of such research irregularities involving for-profit companies, such as the refusal to provide all study data to the study team,2 reporting only 6 months of data in a trial designed to have 12 months of data as the primary outcome3; incomplete reporting of serious adverse events4-5; and concealing clinical trial data showing harm.6
Once again, she is the Queen of Quotes:
the degree I hold is an MD, not an MDeity; I have no ability to know what is in the minds, hearts, or souls of authors. Furthermore, I do not have, nor desire to have, the resources of law enforcement agencies, but I do know that the accuracy of lie detector tests is questionable.
Source.
Now comes the potential problem. In some instances, the marketing goal of a company dominates the scientific aspect of the company-funded research. There have been a number of high-profile examples of such research irregularities involving for-profit companies, such as the refusal to provide all study data to the study team,2 reporting only 6 months of data in a trial designed to have 12 months of data as the primary outcome3; incomplete reporting of serious adverse events4-5; and concealing clinical trial data showing harm.6
Once again, she is the Queen of Quotes:
the degree I hold is an MD, not an MDeity; I have no ability to know what is in the minds, hearts, or souls of authors. Furthermore, I do not have, nor desire to have, the resources of law enforcement agencies, but I do know that the accuracy of lie detector tests is questionable.
Source.
Barr - Plan B: OTC at long last!

The FDA are to allow Barr Pharmaceuticals Inc. to sell its Plan B emergency-contraceptive pill without a prescription to women age 18 and older.
The decision comes more than four years after a company later acquired by Barr first requested to sell the emergency-contraceptive pill, also known as the "morning-after" pill, on an over-the-counter basis.
The decision could clear the way for Senate approval of Acting FDA Commissioner Andrew von Eschenbach as full FDA chief; his approval has been held up over the Plan B issue.
Insider's view: at long last! Hooray!
Drug Reps - an endangered species?

Are drug reps a "dying breed"?
After seeing their numbers more than triple – from 30,000 in 1985 to 100,000 in 2005, according to the Amundsen Group consulting firm – at last the ranks of drug reps are starting to thin.
In New Jersey – home of pharmaceutical giants Wyeth and Merck – sales and marketing employment at the biggest drug companies fell from 23 percent of their overall workforce in 2004 to 16 percent in 2005.
Both Wyeth and Merck have said rising criticism of the industry's long-standing strategy of smothering doctors with sales pitches has also prompted them to scale back their sales forces.
"Doctors are so incredibly busy with managing their patient loads, they really don't have free time" to listen to sales representatives, said Joe Mahady, president of Wyeth's American and Global Businesses.
That wasn't the case 10 or 15 years ago, when the industry began ratcheting up the numbers of sales reps in the belief that the more times a doctor was approached about a given drug, the more likely that doctor was to prescribe it.
For many years, that strategy worked, Mr. Mahady said, leading to the explosion in the numbers of sales reps nationwide.
"It was a ground war," he said.
During this period, the practice of "mirroring" a doctor became popular.
"Mirroring" is when several reps from the same company visit the same doctor to make the same pitch for the same drug again and again.
Wyeth has reduced its full-time sales force to 4,500, down 500 from a year ago.
Meanwhile, Merck chief executive Richard C. Clark has been outspoken in explaining the company's decision to save money by reducing its sales force and increasing the efficiency of the reps who remain.
At meetings with analysts and shareholders in recent months, Mr. Clark has said the move was prompted primarily by negative feedback from physicians.
More
Singa-longa-docs - it's for charity, mate!

Paracetamoxyfrusebendroneomycin
and
The Drugs Song
Link to the artistes . They also have a blog. Buy the CD .
Hat tip: Dr Joan Bushwell
Oh, and by the way, they a have a most excellent rant about the "joys" of travelling on London Underground . Sung from the heart and with words that could make almost anyone blush! Be warned!
Apotex / Plavix - How to make $2 billion in 180 days

Here's a great review of the current situation.
By getting in on the market sooner than expected, Apotex now has 180 days to sell its generic version of Plavix without competition from another generic unless BMS and Sanofi win an injunction to halt sales.
We should hear about that next week.
Even if the decision goes against Apotex, their negotiated contract with BMS and Sanofi limits any compensation; they will still make money!
CEO Barry Sherman figures sales could reach $2-billion during the 180 days, making it the most successful generic launch ever.
Since Aug. 8, Apotex has shipped more than 300 million pills.
The generic drug's market share accounts for more than 60 per cent of total Plavix prescriptions and 74 per cent of new prescriptions.
Mr Sherman is in line for PharmaGossip's Person of the Year Award!
GSK - JP Garnier speaks openly about Big Pharma's Big Problem

GSK's soon to retire chief executive Jean-Pierre Garnier must be a little "demob happy", as he has started speaking very frankly about Big Pharma's Big Problem.
He has told Manager Magazin that he foresees several pharmaceutical companies folding in the next few years because of increasing outlays for research.
'Of the 15 pharma companies that have a significant role in the world, only a handful will remain,' Garnier said in an interview to be published on Friday.
'Hardly any of the large companies are in the position right now to invest enough money into research,' Garnier continued.
A plug for GSK here, who have "upped" their R&D spending more than other Big Pharmas.
Garnier said pharmaceutical companies invest too much money in advertising and marketing at the expense of more research, development and production.
This follows on from Lilly's CEO Sid Taurel, who has also publically admitted the size of the problem ans the most likely solution.
Insider's view: At last! The reality of the situation is being acknowledged. I wonder if we are being "softened up for some dramatic news"!
Forbes
Wednesday, August 23, 2006
Dr. John Abramson on Big Pharma
| Primary care physician John Abramson takes aim at the pharmaceutical industry in "Overdosed America," his recent book that questions the biased and, at times, faulty research that leads doctors to over-prescribe medicines to their patients in this address to the Catfish Club and City Club of San Diego. Series: "City Club Presents" | |
Another "Side Effects" movie
Link to iFilm
Not the Kathleen Slattery-Moschkau movie that's here.
This 'Side Effects' is a 17 minute darkly humorous tale of a healthy young man who gets seduced by the pharmaceutical industry, and finds himself embroiled in a catch 22 of side effects - popping one pill to get rid of the pernicious side effects of another. Ultimately Zubi (Csaba S. Lucas) finds himself recycled by the system both professionally and personally.
It's made by http://www.repertoriumfilms.com/
Not the Kathleen Slattery-Moschkau movie that's here.
This 'Side Effects' is a 17 minute darkly humorous tale of a healthy young man who gets seduced by the pharmaceutical industry, and finds himself embroiled in a catch 22 of side effects - popping one pill to get rid of the pernicious side effects of another. Ultimately Zubi (Csaba S. Lucas) finds himself recycled by the system both professionally and personally.
It's made by http://www.repertoriumfilms.com/
Merck - Arcoxia: Nice try but no MEDAL yet!
Arcoxia (etoricoxib) was to be Merck's follow-up to Vioxx.
"After four years of conducting this arthritis study program in more than 34,000 patients, we are looking forward to sharing the full MEDAL Program results in the future," said Dr. Peter S. Kim, president, Merck Research Laboratories.
Yeah, right. Sure thing Peter.
As Forbes say:
"The Arcoxia results will represent yet another minefield for Merck, now operating under the shadow of more than 11,000 Vioxx lawsuits that could cost it many billions of dollars. Even if the giant study, called MEDAL, shows no link between Arcoxia and heart problems, critics are likely to claim the study was designed in a way that hides the drug's risks.
But with no other study of a Vioxx-like drug's heart risk expected for at least three years, researchers who are hungry for leads as to why and how arthritis drugs lead to heart problems are likely to pore over the data."
The MEDAL Program was a prospectively designed clinical program combining CV safety data from three trials - the MEDAL, EDGE and EDGE II studies.
The MEDAL study, the longest of the three studies, was an event-driven CV outcomes study that included data in more than 23,000 OA and RA patients. The primary hypothesis of the MEDAL Program was that in the treatment of patients with OA and RA, Arcoxia 60 or 90 mg would be non-inferior or "no worse than" diclofenac 150 mg daily based on confirmed thrombotic CV events.
Thus, Arcoxia met its main goal of causing no more blood clot-related heart attacks than diclofenac, but more patients taking Arcoxia withdrew from the trial due to serious side effects. According to this preliminary analysis, the incidence of patients withdrawing from the trial due to side effects related to high blood pressure, edema and congestive heart failure was significantly higher for Arcoxia than for diclofenac.
Why diclofenac?
Unfortunately, there is a "distinct possibility" that MEDAL will not give any clear answers about the Cox -2 drugs and cardiovascular risk, according to Steven Nissen, a Cleveland Clinic cardiologist who was one of Vioxx's earliest critics.
Nissen frets that Merck is comparing Arcoxia to diclofenac, a drug that itself is thought to be a Cox-2 inhibitor that poses some cardiovascular risk.
In the lab, it behaves much like Celebrex. Moreover, he says, the MEDAL study takes into account not only heart attacks, strokes and cardiovascular events, but also clots in the veins and lungs, which might not be the result of Cox-2 drugs at all.
To further cloud the matter, heart attacks and other cardiovascular problems will only be counted in the first 14 days after patients stop their painkiller. In an editorial in the American Heart Journal, where the MEDAL design was recently published, Curt Furberg of Wake Forest University wrote that there is "no scientific support" for handling the data in this way. Furberg also wrote that the safety data on Celebrex are "disturbing."
So, the devil will lie in the details. And we await the "picking over" of the detailed data, once it has been revealed!
"After four years of conducting this arthritis study program in more than 34,000 patients, we are looking forward to sharing the full MEDAL Program results in the future," said Dr. Peter S. Kim, president, Merck Research Laboratories.
Yeah, right. Sure thing Peter.
As Forbes say:
"The Arcoxia results will represent yet another minefield for Merck, now operating under the shadow of more than 11,000 Vioxx lawsuits that could cost it many billions of dollars. Even if the giant study, called MEDAL, shows no link between Arcoxia and heart problems, critics are likely to claim the study was designed in a way that hides the drug's risks.
But with no other study of a Vioxx-like drug's heart risk expected for at least three years, researchers who are hungry for leads as to why and how arthritis drugs lead to heart problems are likely to pore over the data."
The MEDAL Program was a prospectively designed clinical program combining CV safety data from three trials - the MEDAL, EDGE and EDGE II studies.
The MEDAL study, the longest of the three studies, was an event-driven CV outcomes study that included data in more than 23,000 OA and RA patients. The primary hypothesis of the MEDAL Program was that in the treatment of patients with OA and RA, Arcoxia 60 or 90 mg would be non-inferior or "no worse than" diclofenac 150 mg daily based on confirmed thrombotic CV events.
Thus, Arcoxia met its main goal of causing no more blood clot-related heart attacks than diclofenac, but more patients taking Arcoxia withdrew from the trial due to serious side effects. According to this preliminary analysis, the incidence of patients withdrawing from the trial due to side effects related to high blood pressure, edema and congestive heart failure was significantly higher for Arcoxia than for diclofenac.
Why diclofenac?
Unfortunately, there is a "distinct possibility" that MEDAL will not give any clear answers about the Cox -2 drugs and cardiovascular risk, according to Steven Nissen, a Cleveland Clinic cardiologist who was one of Vioxx's earliest critics.
Nissen frets that Merck is comparing Arcoxia to diclofenac, a drug that itself is thought to be a Cox-2 inhibitor that poses some cardiovascular risk.
In the lab, it behaves much like Celebrex. Moreover, he says, the MEDAL study takes into account not only heart attacks, strokes and cardiovascular events, but also clots in the veins and lungs, which might not be the result of Cox-2 drugs at all.
To further cloud the matter, heart attacks and other cardiovascular problems will only be counted in the first 14 days after patients stop their painkiller. In an editorial in the American Heart Journal, where the MEDAL design was recently published, Curt Furberg of Wake Forest University wrote that there is "no scientific support" for handling the data in this way. Furberg also wrote that the safety data on Celebrex are "disturbing."
So, the devil will lie in the details. And we await the "picking over" of the detailed data, once it has been revealed!
A date for your diary - Big Pharma B.S. Festival
Jacob Fleming Group has announced that Le Meridien Hotel in Vienna will host speakers and delegates at conference on Linking Corporate Communication with Business Performance on November 14 and 15, 2006.
The event will examine issues like how to improve productivity through communication and how to apply proactive aproach towards media.
“Building and protecting corporate reputation is the biggest challenge which life science companies face today. Effective and responsible communication is the key to achieve this,” said Marina Manojlovic, Senior Conference Producer of the Jacob Fleming Group.
“Case studies on blogging, on-line channels or internal communication will enable delegates to understand how to drive business performance through effective corporate communication.”
Blogging!?
Insider might sign up. Well, there should be some great Christmas shopping opportunities!
The event will examine issues like how to improve productivity through communication and how to apply proactive aproach towards media.
“Building and protecting corporate reputation is the biggest challenge which life science companies face today. Effective and responsible communication is the key to achieve this,” said Marina Manojlovic, Senior Conference Producer of the Jacob Fleming Group.
“Case studies on blogging, on-line channels or internal communication will enable delegates to understand how to drive business performance through effective corporate communication.”
Blogging!?
Insider might sign up. Well, there should be some great Christmas shopping opportunities!
Ricky Gervais as David Brent - Microsoft "Office Values" videos

Technical issues.
http://video.google.com/videosearch?q=ricky+gervais+microsoft
The above link should get you to them on Google Video. There have been some strange problems with the links for this to GV.
If all else fails , go to Google Video and search "Ricky Gervais Microsoft" and they will come up.
Enjoy!!
PS dont miss the "out takes" at the end of each one! Ricky laughs like a hyena!!
Moore's "Sicko" flick is worrying Big Pharma's spinmeisters
Michael Moore’s upcoming 2007 documentary “Sicko”, aimed at the $1.5 trillion healthcare and pharmaceutical industry, has mobilized many companies within the medical industry to try to discredit both Moore and the film, AdAge.com reports.
"A review of America's health-care system should be balanced, thoughtful and well-researched to pin down what works and what needs to be improved," said Ken Johnson, senior VP for the Pharmaceutical Research and Manufacturers of America. "You won't get that from Michael Moore."
Added a spokesman for one of the top 10 pharma companies: "We expect it will be one-sided and biased, just like his other documentaries." Several other pharmaceutical makers did not return calls for comment.
But Pfizer, AstraZeneca and GlaxoSmithKline all advised their employees last year not to speak to Mr. Moore when he began his research for "Sicko." It is not known whether any HMOs or drug companies will appear in the film.
"We were approached, but declined," said a spokeswoman for a second top-10 drugmaker. "Frankly, as much as we felt like we wanted to get our message across, in the end we didn't want to subject ourselves to the editing process."
Insider cant wait to see the movie.
Until then, his advice to anyone suffering under a current healthcare nightmare situation is to "mention Mike and Sicko" ...... and suddenly all your problems will go away!
"A review of America's health-care system should be balanced, thoughtful and well-researched to pin down what works and what needs to be improved," said Ken Johnson, senior VP for the Pharmaceutical Research and Manufacturers of America. "You won't get that from Michael Moore."
Added a spokesman for one of the top 10 pharma companies: "We expect it will be one-sided and biased, just like his other documentaries." Several other pharmaceutical makers did not return calls for comment.
But Pfizer, AstraZeneca and GlaxoSmithKline all advised their employees last year not to speak to Mr. Moore when he began his research for "Sicko." It is not known whether any HMOs or drug companies will appear in the film.
"We were approached, but declined," said a spokeswoman for a second top-10 drugmaker. "Frankly, as much as we felt like we wanted to get our message across, in the end we didn't want to subject ourselves to the editing process."
Insider cant wait to see the movie.
Until then, his advice to anyone suffering under a current healthcare nightmare situation is to "mention Mike and Sicko" ...... and suddenly all your problems will go away!
Tuesday, August 22, 2006
Pfizer - Look what your sales and marketing have done to Medpundit
"The Summer of My Disenchantment: I am ready to throw in the towel and admit defeat. The drug companies have won. They've suceeded in dominating the debate about which medicine to use for what and when. Professional judgement and experience, and scientific evidence have failed to check their promotional assaults. They've won the battle, and maybe the war. "
Why? Who? How?
Read it and weep!
Insider is a huge fan of Medpundit. Look at what Pfizer (and the others) have done to her spirit!
He will neither forgive or forget.
Why? Who? How?
Read it and weep!
Insider is a huge fan of Medpundit. Look at what Pfizer (and the others) have done to her spirit!
He will neither forgive or forget.
Sanofi Aventis - Astellas sidle away from Ketek

Lots of back stories here.
Now, Japanese Big Pharma company Astellas have retreated from its marketing alliance with Sanofi-Aventis for the embattled antibiotic Ketek (telithromycin), a decision that follows recent FDA investigations into its safety.
Sanofi-Aventis will, from October 1, market the drug alone in Japan.
Ketek was launched in December 2003 for treating various respiratory tract infections, including acute bronchitis and pneumonia, as well as tonsillitis, sinusitis and pharyngitis/laryngitis.
There was no mention as to why Astellas decided to back away from its alliance for Ketek, but the drug has come under increasing scrutiny in the USA and Europe.
Most recently, Sanofi-Aventis suspended a clinical trial in children and - although it says it was unrelated to the safety issues raised - senior US politicians are saying it should never have been approved because of unanswered questions relating to its safety and efficacy.
The FDA is considering changing the labelling after a review linked the drug to 12 cases of liver failure and four deaths. It is also under review in Europe.
Source: PharmaTimes
Toddler T-Shirt Slogans - LOL
Toddler T-Shirt Slogans.
BY DARRYL BERGER
Ask me about the C-section.
More of a tit man, thanks.
Still pissed about missing the millennium.
I buried my heart at Legoland.
Waiting for Godot.
Don't let Tony Danza touch me.
Stop the war. Already.
Stunt double for Katie Holmes's baby.
Property of Child and Family Services
Glad those stairs were carpeted.
Slap me if you love Jesus.
Not quite getting this whole "MILF" phenomenon.
I beheld then because of the voice of the great words which the horn spake: I beheld even till the beast was slain, and his body destroyed, and given to the burning flame.
Daddy didn't want me.
Ask me about the extra digit.
Grandma won't shut up.
Hat tip: McSweeneys
BY DARRYL BERGER
Ask me about the C-section.
More of a tit man, thanks.
Still pissed about missing the millennium.
I buried my heart at Legoland.
Waiting for Godot.
Don't let Tony Danza touch me.
Stop the war. Already.
Stunt double for Katie Holmes's baby.
Property of Child and Family Services
Glad those stairs were carpeted.
Slap me if you love Jesus.
Not quite getting this whole "MILF" phenomenon.
I beheld then because of the voice of the great words which the horn spake: I beheld even till the beast was slain, and his body destroyed, and given to the burning flame.
Daddy didn't want me.
Ask me about the extra digit.
Grandma won't shut up.
Hat tip: McSweeneys
Walgreens - "Take Care Clinics" coming to Chicago
Chain stores, supermarkets and shopping malls are shaking up medicine by opening health clinics that are remarkably consumer friendly.
The walk-in clinics require no appointment and little or no waiting for treatment of minor ailments such as flu, bladder infections and sprains. They're open nights and weekends.
Prices are posted, and the bills are surprisingly reasonable.
At least nine companies, with names such as MinuteClinic and RediClinic, have opened hundreds of retail clinics in the last few years.
TAKE CARE CLINICS
The clinics will offer these services when they open at 20 Chicago area Walgreens stores this fall.
Here's the "menu":
Respiratory illnesses: Bronchitis, colds, coughs, ear infections, flu, laryngitis, sinus infections, sore throat, strep throat, upper respiratory infections.
Common treatments: Bladder infections, diarrhea-nausea-vomiting, early Lyme disease, fever less than 72 hours, head lice, infected cuticles, mononucleosis, pink eye and sties, scalp rash, seasonal allergies, swimmer's ear, swimmer's itch.
Diagnostic screenings: Blood pressure, blood sugar, tuberculosis, pregnancy.
Minor injuries: Abrasions, minor burns, splinters, sprains and strains, staple and suture removals.
Vaccinations: Flu, hepatitis B, meningitis, tetanus/ pertussis booster, tetanus booster, travel vaccines.
Sports and camp physicals.
More
The walk-in clinics require no appointment and little or no waiting for treatment of minor ailments such as flu, bladder infections and sprains. They're open nights and weekends.
Prices are posted, and the bills are surprisingly reasonable.
At least nine companies, with names such as MinuteClinic and RediClinic, have opened hundreds of retail clinics in the last few years.
TAKE CARE CLINICS
The clinics will offer these services when they open at 20 Chicago area Walgreens stores this fall.
Here's the "menu":
Respiratory illnesses: Bronchitis, colds, coughs, ear infections, flu, laryngitis, sinus infections, sore throat, strep throat, upper respiratory infections.
Common treatments: Bladder infections, diarrhea-nausea-vomiting, early Lyme disease, fever less than 72 hours, head lice, infected cuticles, mononucleosis, pink eye and sties, scalp rash, seasonal allergies, swimmer's ear, swimmer's itch.
Diagnostic screenings: Blood pressure, blood sugar, tuberculosis, pregnancy.
Minor injuries: Abrasions, minor burns, splinters, sprains and strains, staple and suture removals.
Vaccinations: Flu, hepatitis B, meningitis, tetanus/ pertussis booster, tetanus booster, travel vaccines.
Sports and camp physicals.
More
Disease Mongering - it makes me sick!
Jonathan Rowe, issues director at Commercial Alert writes:
It is an old saying in the advertising trade that you sell the problem, not the solution. That helps explain why the media today are awash with images of disease. Erectile dysfunction, depression, stress, attention deficit disorder, on and on - you can't escape them and the sense of looming peril that they conjure up.
Politicians sell terror and fear; pharmaceutical companies sell disease. Every state and stage of existence has become a pathology in need of pharmaceutical "intervention," and life itself is a petri dish of biochemical deficiency and need. Shyness is now "social anxiety disorder." A twitchy tendency has become "restless leg syndrome."
Three decades ago the head of Merck dreamed aloud of the day when the definition of disease would be so broad that his company could "sell to everyone," like chewing gum.
That day is rapidly approaching, if it's not already here. "We're increasingly turning normal people into patients," said Dr. Lisa M. Schwartz of the Dartmouth Medical School. "The ordinary experiences of life become a diagnosis, which makes healthy people feel like they're sick."
In one sense, the ads have been successful. The Kaiser Family Foundation found that every dollar drug companies spend on ads brings more than four dollars in additional sales. But for most others, the result has been soaring medical insurance costs, toxic side effects, and new tensions between doctors and patients, who increasingly badger doctors for the drugs they've seen on TV.
One study found that 30 percent of Americans have made these demands. A Minnesota doctor complained recently that patients now push him for sleep medications "when maybe they just need to go to bed on a more regular basis."
But perhaps the worst part is that prescription drug ads have immersed us all in a pervasive drug culture that seems to have no boundaries. We are being reduced to helpless "consumers" who have no capacity to deal with challenges other than by taking a pill. Last month Tim Pawlenty, the Republican governor of Minnesota, called for a moratorium on prescription drug ads.
It's about time.
For most of the past half century, there were tight restrictions on the general advertising of prescription drugs. These require doctors' guidance for a reason; so why should Madison Avenue get involved? But under heavy pressure from the drug and advertising industries, the government backed down in the late 1990s, and that started the tsunami.
Spending on drug ads for the general public more than tripled between 1996 and 2001. It is now some $4 billion a year, which is more than twice what McDonald's spends on ads. In 1994, the typical American had seven prescriptions a year, which is no small number. By 2004, that was up to 12 a year.
Homebuilders are touting medicine cabinets that are "triple-wide."
The industry says this is all about "educating" the consumer. But an ad executive was more candid when he said - boasted, really - that the goal is to "drive patients to their doctors." Reuters Business Insight, a publication for investors, explained that the future of the industry depends on its ability to "create new disease markets." "The coming years," it said, "will bear greater witness to the corporate-sponsored creation of disease."
The Kaiser study found that drug ads increase sales for entire categories of drugs, not just the one in question. The ads really are selling the disease more than a cure.
Advertising is just one way the industry has sought to accomplish this goal. It also funds patient advocacy groups such as Children With Attention Deficit Disorder (CHADD), and doctors who push for expanded definitions of disease, among a host of other things. (When the definition of ADD expanded in the 1980s, the number of kids tagged with this problem increased by 50 percent.)
But advertising is the most pervasive and aggressive way of selling sickness. It also is the hardest to justify. Medicine is supposed to be about science, not huckstering; about healing people, not persuading more of them that they are sick. There are far better ways to inform the public about health issues than to spend billions of dollars a year pushing pills.
This is why more than 200 medical school professors recently called for an end to prescription drug ads, and why close to 40 health and seniors groups have joined them. Even the American Medical Association, many members of which have close ties to the pharmaceutical industry, has urged restrictions.
Washington should listen to these doctors.
As Governor Pawlenty put it, we need to put "the decisionmaking back where it should be - on an informed basis between the patient and the doctor."
It is an old saying in the advertising trade that you sell the problem, not the solution. That helps explain why the media today are awash with images of disease. Erectile dysfunction, depression, stress, attention deficit disorder, on and on - you can't escape them and the sense of looming peril that they conjure up.
Politicians sell terror and fear; pharmaceutical companies sell disease. Every state and stage of existence has become a pathology in need of pharmaceutical "intervention," and life itself is a petri dish of biochemical deficiency and need. Shyness is now "social anxiety disorder." A twitchy tendency has become "restless leg syndrome."
Three decades ago the head of Merck dreamed aloud of the day when the definition of disease would be so broad that his company could "sell to everyone," like chewing gum.
That day is rapidly approaching, if it's not already here. "We're increasingly turning normal people into patients," said Dr. Lisa M. Schwartz of the Dartmouth Medical School. "The ordinary experiences of life become a diagnosis, which makes healthy people feel like they're sick."
In one sense, the ads have been successful. The Kaiser Family Foundation found that every dollar drug companies spend on ads brings more than four dollars in additional sales. But for most others, the result has been soaring medical insurance costs, toxic side effects, and new tensions between doctors and patients, who increasingly badger doctors for the drugs they've seen on TV.
One study found that 30 percent of Americans have made these demands. A Minnesota doctor complained recently that patients now push him for sleep medications "when maybe they just need to go to bed on a more regular basis."
But perhaps the worst part is that prescription drug ads have immersed us all in a pervasive drug culture that seems to have no boundaries. We are being reduced to helpless "consumers" who have no capacity to deal with challenges other than by taking a pill. Last month Tim Pawlenty, the Republican governor of Minnesota, called for a moratorium on prescription drug ads.
It's about time.
For most of the past half century, there were tight restrictions on the general advertising of prescription drugs. These require doctors' guidance for a reason; so why should Madison Avenue get involved? But under heavy pressure from the drug and advertising industries, the government backed down in the late 1990s, and that started the tsunami.
Spending on drug ads for the general public more than tripled between 1996 and 2001. It is now some $4 billion a year, which is more than twice what McDonald's spends on ads. In 1994, the typical American had seven prescriptions a year, which is no small number. By 2004, that was up to 12 a year.
Homebuilders are touting medicine cabinets that are "triple-wide."
The industry says this is all about "educating" the consumer. But an ad executive was more candid when he said - boasted, really - that the goal is to "drive patients to their doctors." Reuters Business Insight, a publication for investors, explained that the future of the industry depends on its ability to "create new disease markets." "The coming years," it said, "will bear greater witness to the corporate-sponsored creation of disease."
The Kaiser study found that drug ads increase sales for entire categories of drugs, not just the one in question. The ads really are selling the disease more than a cure.
Advertising is just one way the industry has sought to accomplish this goal. It also funds patient advocacy groups such as Children With Attention Deficit Disorder (CHADD), and doctors who push for expanded definitions of disease, among a host of other things. (When the definition of ADD expanded in the 1980s, the number of kids tagged with this problem increased by 50 percent.)
But advertising is the most pervasive and aggressive way of selling sickness. It also is the hardest to justify. Medicine is supposed to be about science, not huckstering; about healing people, not persuading more of them that they are sick. There are far better ways to inform the public about health issues than to spend billions of dollars a year pushing pills.
This is why more than 200 medical school professors recently called for an end to prescription drug ads, and why close to 40 health and seniors groups have joined them. Even the American Medical Association, many members of which have close ties to the pharmaceutical industry, has urged restrictions.
Washington should listen to these doctors.
As Governor Pawlenty put it, we need to put "the decisionmaking back where it should be - on an informed basis between the patient and the doctor."
BMS - Apotex: The Case of the Short Dated Clopidogrel
 From the FT
From the FTThe patent fight over Plavix has taken on the characteristics of a pharmaceuticals industry 'whodunnit' mystery.
The case over the world's second best-selling medicine highlights an aggressive generic drug industry pressing branded drugmakers with bold tactics.
It has emerged that a previously unknown inventory build-up of a generic version of Plavix spurred Bristol-Myers Squibb and Sanofi-Aventis's negotiations with Apotex, maker of the generic version, over the threat to their blood-thinner.
Expiry dates on generic Plavix on shelves now also confirm that Apotex was making the drug late last year. Generic Plavix stocked in an outpatient pharmacy at a university hospital in Chicago carries an expiry date of October 2, 2007.
Lawyers for the companies have asserted that the typical shelf life of generic Plavix is two years. According to them, the generic Plavix in question was made in October last year.
"What does it all mean Holmes?"
"Go here, Watson, and read it for youself!"
Monday, August 21, 2006
Troops on acid - the way forward?
Insider shared with you today that, according to a questionnaire he completed, his personality is like acid (LSD) .
Go here to see an old recording of British troops being given LSD.
Looks like fun!
Go here to see an old recording of British troops being given LSD.
Looks like fun!
Today is "Tell Another Person About PharmaGossip Day"

Dear Readers,
Thank you so much for your loyalty and discerning good taste in choosing PharmaGossip as your part of your daily blogging ritual.
It warms Insider's little heart to know that so many of you are such firm fans.
Could Insider now ask a small favour, please?
Could you each find someone to share your little secret with?
This would help PharmaGossip to continue its miraculous growth rate and continue to help "spread the word" around the blogosphere.
Please do "your bit" and tell someone today about "PharmaGossip"!
Thanking you in anticipation,
Jack
Avastin and Erbitux - NICE, but not nice enough!
Two new but expensive bowel cancer treatments should not be used in the UK's NHS, the country's cost-effectiveness watchdog NICE said today.
The National Institute for Health and Clinical Excellence (NICE), however, decided the high cost of Avastin and Erbitux meant their use was not "compatible with the best use of NHS (National Health Service) resources".
Welcome to the real world, Big Pharma.
Reuters
The National Institute for Health and Clinical Excellence (NICE), however, decided the high cost of Avastin and Erbitux meant their use was not "compatible with the best use of NHS (National Health Service) resources".
Welcome to the real world, Big Pharma.
Reuters
How fast are Apotex making generic Plavix tablets?
7,142 a minute, that's how fast!
Meanwhile, the judge hearing the high-stakes "Humpty Dumpty" arguments, Sidney Stein of the U.S. District Court for the Southern District in Manhattan, is not expected to rule on the matter on Monday, his law clerk said earlier this morning.
"His decision will come later," the court clerk said, declining to speculate when it would be delivered.
Meanwhile, the judge hearing the high-stakes "Humpty Dumpty" arguments, Sidney Stein of the U.S. District Court for the Southern District in Manhattan, is not expected to rule on the matter on Monday, his law clerk said earlier this morning.
"His decision will come later," the court clerk said, declining to speculate when it would be delivered.
Looks like adverlicio.us is going to be busy
http://adverlicio.us/pharma is where you can see all the on-line drug ads Big Pharma currently pump out.
Looks like they are gonna get busier.
"US pharmaceutical giants - in common with other industries - are shifting their advertising focus to online marketing, more closely targeted at specific consumers. "
According to research firm eMarketer the pharma sector will up online adspend by around 25% this year to $780 million (€608m; £414m) and by 2008, it predicts, this figure will rise to $1.3 billion.
More than 31 million US patients now turn to the web for health information. Comments eMarketer senior analyst Lisa Phillips: "Marketers are focusing more on providing information and a place for communities to gather on websites geared to specific conditions and questions about treatment."
Looks like they are gonna get busier.
"US pharmaceutical giants - in common with other industries - are shifting their advertising focus to online marketing, more closely targeted at specific consumers. "
According to research firm eMarketer the pharma sector will up online adspend by around 25% this year to $780 million (€608m; £414m) and by 2008, it predicts, this figure will rise to $1.3 billion.
More than 31 million US patients now turn to the web for health information. Comments eMarketer senior analyst Lisa Phillips: "Marketers are focusing more on providing information and a place for communities to gather on websites geared to specific conditions and questions about treatment."
Insider is acidic
| Your Personality Is Like Acid |
 A bit wacky, you're very difficult to predict. One moment you're in your own little happy universe... And the next, you're on a bad trip to your own personal hell! |
Wyeth - Prempro: now where have we heard that before?
"We plan to fight every case."
So say Wyeth at the start of the first "fountain of youth" case against their HRT product Prempro.
Sounds a bit like the Merck (Vioxx) lawyers doesnt it?
This first of more than 5,000 suits involving Wyeth's hormone replacement drugs is scheduled to go to trial today in U.S. District Court in Little Rock, Arkansas. A second is scheduled to begin in September in a Pennsylvania state court in Philadelphia.
Shannon Pettypiece and Sophia Pearson of Bloomberg News have an excellent briefing report in the International Herald Tribune.
The "every case" defence is standard for this type of situation.
Merck are still stonewalling with Vioxx. They will continue to do this until they get a feel for how much they might have to pay out in total if they agreed to settle.
This is where Wyeth have more "experience".
They had a similar problem in the past with the fenfluramine (Pondimin/Redux) part of the "phen-fen" diet drug issue.
That cost them $21 billion!
Insider's view: it will depend on how well the plaintiffs use Wyeth's CEO Robert Essner's own words and other marketing materials to show that they oversold the product.
So say Wyeth at the start of the first "fountain of youth" case against their HRT product Prempro.
Sounds a bit like the Merck (Vioxx) lawyers doesnt it?
This first of more than 5,000 suits involving Wyeth's hormone replacement drugs is scheduled to go to trial today in U.S. District Court in Little Rock, Arkansas. A second is scheduled to begin in September in a Pennsylvania state court in Philadelphia.
Shannon Pettypiece and Sophia Pearson of Bloomberg News have an excellent briefing report in the International Herald Tribune.
The "every case" defence is standard for this type of situation.
Merck are still stonewalling with Vioxx. They will continue to do this until they get a feel for how much they might have to pay out in total if they agreed to settle.
This is where Wyeth have more "experience".
They had a similar problem in the past with the fenfluramine (Pondimin/Redux) part of the "phen-fen" diet drug issue.
That cost them $21 billion!
Insider's view: it will depend on how well the plaintiffs use Wyeth's CEO Robert Essner's own words and other marketing materials to show that they oversold the product.
Sunday, August 20, 2006
FDA - move over Big Pharma, here comes Big Tobacco
A federal court ruling branding cigarette makers as racketeers has rekindled calls for Congress to impose tough new restrictions on the tobacco industry.
U.S. District Judge Gladys Kessler rebuked Big Tobacco in her ruling Thursday, saying they had conspired for decades to deceive the public about smoking's hazards and pitched their products to youngsters and lied about it.
Kessler said she could not require the industry to pay for a multibillion dollar quit-smoking campaign, citing an interim ruling, but she suggested federal lawmakers could do more to stop the industry from addicting new generations of smokers.
Sens. Frank Lautenberg, D-N.J., and Edward Kennedy, D-Mass., predicted Kessler's ruling would help boost the chances for bringing the loosely regulated tobacco industry under the control of the FDA.
AP
U.S. District Judge Gladys Kessler rebuked Big Tobacco in her ruling Thursday, saying they had conspired for decades to deceive the public about smoking's hazards and pitched their products to youngsters and lied about it.
Kessler said she could not require the industry to pay for a multibillion dollar quit-smoking campaign, citing an interim ruling, but she suggested federal lawmakers could do more to stop the industry from addicting new generations of smokers.
Sens. Frank Lautenberg, D-N.J., and Edward Kennedy, D-Mass., predicted Kessler's ruling would help boost the chances for bringing the loosely regulated tobacco industry under the control of the FDA.
AP
Cleveland Clinic carpets compromised cardiologist's Cordis Corp. cover-up
The Cleveland Clinic has removed a cardiologist for failing to disclose his financial interest in a stroke-preventing device that he invented and reviewed positively in a national study, a clinic spokeswoman said Friday.
Dr. Jay Yadav and others sold rights to the device, Angioguard, to Cordis Corp., a division of New Brunswick, N.J.-based Johnson & Johnson, in 1999 in a deal worth $40 million, plus royalties, The Plain Dealer reported in Friday’s editions.
Yadav failed to properly disclose that fact to the clinic and the FDA during clinical reviews of Angioguard, the newspaper reported.
More
Dr. Jay Yadav and others sold rights to the device, Angioguard, to Cordis Corp., a division of New Brunswick, N.J.-based Johnson & Johnson, in 1999 in a deal worth $40 million, plus royalties, The Plain Dealer reported in Friday’s editions.
Yadav failed to properly disclose that fact to the clinic and the FDA during clinical reviews of Angioguard, the newspaper reported.
More
BMS - Plavix: call Mr. H. Dumpty!

"You can never put this Humpty Dumpty back together if it's not stopped," Evan Chesler, a lawyer for Bristol-Myers and Sanofi-Aventis, said as the Plavix hearing on the companies' request began in U.S. District Court in New York.
Like Jon Stewart on The Daily Show says: "sometimes the jokes just write themselves!"
See for yourself.
Saturday, August 19, 2006
BMS - Plavix: Dolan - a week is a long time in Big Pharma
LAST WEEK:
James Robinson, BMS's chairman, has said that CEO Peter Dolan has the company's unwavering support."Peter has done a superb job in turning around the company, from developing a new management team, to a pipeline considered to be one of the best in the industry, to the recent approvals which bring innovative new medicines to patients in need," Robinson said in a statement the company released Friday. " He has the full and complete confidence of the board."
THIS WEEK:
Directors of Bristol-Myers Squibb are open to replacing CEO Peter Dolan over his mishandling of a failed settlement to delay generic competition to Plavix, The Wall Street Journal reported Friday, citing an unnamed source. Shares have fallen 20% over the past three weeks after a criminal probe started, state attorneys general rejected the proposed deal and Apotex launched a generic Plavix. According to the report, the board has not found any evidence of misconduct, but the failed settlement threatens a large chunk of its annual $3.8 billion in Plavix revenue.
James Robinson, BMS's chairman, has said that CEO Peter Dolan has the company's unwavering support."Peter has done a superb job in turning around the company, from developing a new management team, to a pipeline considered to be one of the best in the industry, to the recent approvals which bring innovative new medicines to patients in need," Robinson said in a statement the company released Friday. " He has the full and complete confidence of the board."
THIS WEEK:
Directors of Bristol-Myers Squibb are open to replacing CEO Peter Dolan over his mishandling of a failed settlement to delay generic competition to Plavix, The Wall Street Journal reported Friday, citing an unnamed source. Shares have fallen 20% over the past three weeks after a criminal probe started, state attorneys general rejected the proposed deal and Apotex launched a generic Plavix. According to the report, the board has not found any evidence of misconduct, but the failed settlement threatens a large chunk of its annual $3.8 billion in Plavix revenue.
Friday, August 18, 2006
BMS - Plavix: Andy Bodnar and The Side Deals
No, not a new band. Just another twist in the whole Apotex coup story.
Canadian generic drugmaker Apotex, accused BMS of attempting to mislead US regulators about secret side-deals in a settlement to end patent litigation over blockbuster drug Plavix, according to court documents yesterday.
Apotex said it alerted the Federal Trade Commission to efforts by a senior BMS executive, Andrew Bodnar, allegedly to deceive the government about verbal agreements outside settlement provisions.
Source: FT
Canadian generic drugmaker Apotex, accused BMS of attempting to mislead US regulators about secret side-deals in a settlement to end patent litigation over blockbuster drug Plavix, according to court documents yesterday.
Apotex said it alerted the Federal Trade Commission to efforts by a senior BMS executive, Andrew Bodnar, allegedly to deceive the government about verbal agreements outside settlement provisions.
Source: FT
Merck - Vioxx: a bad day at Whitehouse Station
 Poor Merck. Thursday August 17th 2006 was a very bad day for the company and their lawyers' "trial by trial" Vioxx defence strategy.
Poor Merck. Thursday August 17th 2006 was a very bad day for the company and their lawyers' "trial by trial" Vioxx defence strategy.First of all they lost a federal case in The Big Easy.
Then, to cap it all, NJ state judge Carol Higbee threw out a previous victory for Merck and ordered a new trial for a postal worker who blamed his heart attack on taking the company's Vioxx pain medicine for two months.
The company said it might appeal that ruling.
Superior Court Judge Higbee ruled that evidence uncovered since the November verdict revealed that Merck withheld information showing heart attacks could come with use of Vioxx for less than 18 months, said the plaintiff's lawyer, Christopher Seeger.
''Merck consistently said throughout the trial that you had to be on Vioxx for 18 months to be at increased risk of a heart attack,'' Seeger said.
''And that was false. They had data that people were having heart attacks within weeks.''
Thursday, August 17, 2006
Lilly - could their "star" prasugrel be collateral damage from the Plavix affair?
Lilly's blood thinner prasugrel has predicted $1.2 billion in sales in 2012 - but probably wont even come to the all important US market until late 2008/early 2009.
It is part of a partnership between Lilly and Daiichi-Sankyo and is Lilly's most important drug still in development.
It was always going to have to compete with generic Plavix (clopidogrel). But the recent Apotex coup has raised the possibility of earlier generic price erosion.
Any "me-too" would have to demonstrate real advantages to be able to command a premium price!
Source
It is part of a partnership between Lilly and Daiichi-Sankyo and is Lilly's most important drug still in development.
It was always going to have to compete with generic Plavix (clopidogrel). But the recent Apotex coup has raised the possibility of earlier generic price erosion.
Any "me-too" would have to demonstrate real advantages to be able to command a premium price!
Source
Merck - Vioxx: score one for the plaintiffs!

Merck failed to warn doctors about the risks of its painkiller Vioxx and must pay a retired FBI agent $50 million to compensate for the heart attack he suffered after taking the drug, a federal jury found Thursday.
The jury also found that Merck & Co. "knowingly misrepresented or failed to disclose" information about the drug to retired FBI agent Gerald Barnett's doctors.
The same jury was to deliberate later in the day on possible punitive damages for Barnett.
Forbes
Omnicare - "I bill dead people!"

In recent public comments, Omnicare Inc. gave the impression that its trouble with Medicaid-fraud watchdogs in Michigan would be cured with a $54 million payout.
Now the pharmaceuticals supplier is watching one of its top executives being dragged into a criminal courtroom.
The 148 criminal fraud charges filed Wednesday against Daniel Lohmeier, president of Omnicare's Specialized Pharmacy Services in Livonia, Mich., were a stunning turn of events in an investigation that began with the discovery of suspicious billings to the state Medicaid program in 2003.
Charges include billing Medicaid for drugs dispensed to 20 dead people.
More.
Subscribe to:
Comments (Atom)











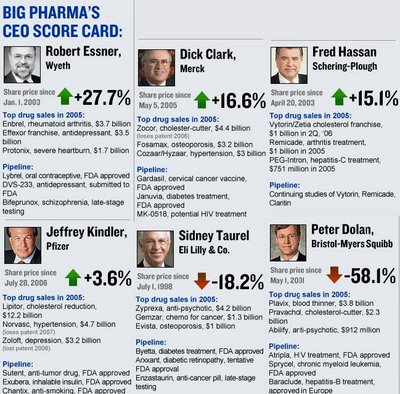



PharmaGossipBETA.png)











